What is Glass?
Glass used in building is sometimes called the SLS (soda-lime-silicate) glass, and it has the following composition:
- silica – the raw material in the form of sand (70%)
- soda – the flux as carbonate and sulphate (approx. 14%)
- lime – stabiliser in the form of limestone (approx. 10%)
- various other oxides such as alumina and magnesia, to improve the physical properties of the glass
Glass, chemically, is actually more like a liquid, but at room temperature it is so viscous or sticky it looks and feels like a solid. At higher temperatures glass gradually becomes softer and more like a liquid. It is this latter property, which allows glass to be poured, blown, pressed and moulded into such a variety of shapes.
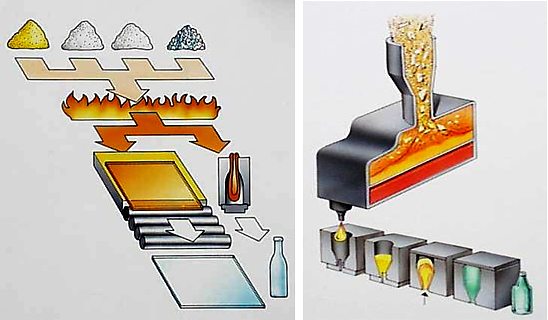
How is Glass made?
Glass is made by melting together several minerals at very high temperatures. Silica in the form of sand is the main ingredient and this is combined with soda ash and limestone and melted in a furnace at temperatures of 1700°C. Other materials can be added to produce different colours or properties. Glass can also be coated, heat-treated, engraved or decorated. Whilst still molten, glass can be manipulated to form packaging, car windscreens, glazing or numerous other products.
Recycling Glass
Glass is 100% recyclable and can be recycled an infinite number of times without quality, strength and/or functionality degradation, therefore it is theoritically unwastable.

